Abstract
Background:
Although the prognosis for children with Hodgkin lymphoma (HL) is excellent, up to 25% of patients experience relapse and require salvage therapy. Event-free survival (EFS) for patients with relapsed or refractory disease (R/R) who require high-dose therapy with autologous stem cell transplantation (ASCT) is between 31-67% (Daw, 2011), and efforts to improve these outcomes are an area of urgent clinical need. Brentuximab vedotin (BV) is an anti-CD30 antibody-drug conjugate combined with monomethyl auristatin E. The phase III AETHERA study (NCT01100502) was a randomized, placebo-controlled trial that demonstrated consolidation with BV following ASCT for adults with R/R HL improved progression-free survival (PFS) in comparison to placebo (5-year PFS 59% vs. 41%) in patients identified as having a high-risk of post-ASCT progression (Moskowitz, 2015, 2018). However, there is limited data on post-ASCT BV consolidation in pediatric patients. Here, we report a retrospective multi-center study of BV as consolidation post-ASCT in pediatric patients with R/R HL.
Patients and Methods:
We performed a retrospective analysis of pediatric patients aged ≤ 21 years who received BV as consolidative therapy following ASCT for the treatment of relapsed/refractory HL. Data was collected on disease risk factors (refractory disease or relapse ≤ 12 months after frontline therapy, best response of partial remission or stable disease to salvage therapy, presence of extranodal involvement, presence of B symptoms, and number of systemic treatments pre-ASCT ≥ 2), treatment history, BV-related toxicity, and response to therapy. The primary endpoint was 3-year EFS. Events were defined as relapse, progression of disease, or death from any cause.
Results:
Seventy patients were identified from 14 academic centers. Eighteen received BV >90 days after ASCT and were excluded due to significant delay in initiation of BV. Of the remaining 52 patients, the median age at diagnosis was 15 years (range 4-21), and the median age at diagnosis of R/R disease was 16 years (range 8-22). Twenty-eight patients (54%) were male, 13 (25%) had B symptoms, 19 (37%) had extranodal disease. Six patients (12%) had primary refractory disease and 24 patients (47%) relapsed <12 months after frontline therapy. The median number of salvage regimens received pre-ASCT was 2 (range 1-7). Forty-two patients (81%) received BV as part of a pre-ASCT salvage regimen, 4 (8%) of whom did not receive BV in the regimen immediately prior to ASCT. Radiation therapy was used pre or post-ASCT in 16 patients (31%). Prior to ASCT, 40 patients (77%) were PET-negative (Deauville 1-3), 11 (21%) were PET-positive (Deauville 4-5), and response in 1 (2%) was unknown.
The median time from ASCT to the start of BV consolidation was 52 days (range 30-90 days). The most frequent adverse events were peripheral neuropathy of any grade (sensory 19/52 [37%]; motor (14/52 [27%]), and cytopenias (grade 3 or 4: (25/52 [48%]), including neutropenia (13/52 [25%]), thrombocytopenia (7/52 [14%]), and anemia (5/52 [10%]). Three patients (6%) experienced infusion-related reactions. The median number of BV cycles completed post-ASCT was 12 (range 1-17), and 16 patients (31%) completed the full 16 cycles of BV. The most common reason for premature termination was adverse effects (22/36 [61%]). Additional reasons included: physician plan for shorter duration (4/36 [11%]), patient/family decision (3/36 [8%]), and progression (1/36 [3%]). Treatment was still ongoing in 3 patients and reason for premature termination was not reported for 17/36 patients (47%).
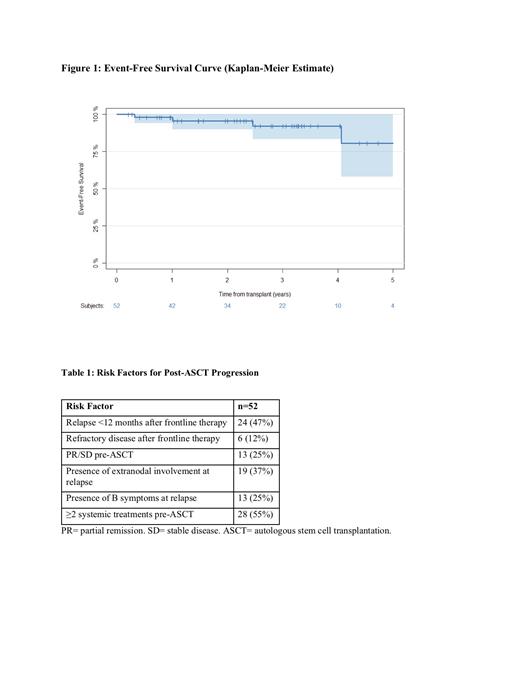
With a median follow of 2.8 years (range 0.1 to 6.25 years) the three-year EFS was 92% [95%CI: 83-100]. Patients with 0-1 risk factors (15/52 [29%]) and ≥ 2 risk factors (37/52 [71%]), had a 3-year EFS of 100% and 90% [95%CI: 79-100], respectively. Fifty patients (96%) are alive with no evidence of disease, while 2 (4%) patients are alive with disease. No deaths have occurred.
Conclusion:
The use of post-ASCT BV consolidation for pediatric patients with R/R HL is associated with a favorable safety and tolerability profile. In this cohort, the combination of salvage therapy, HD-chemotherapy/ASCT, and BV consolidation resulted in extremely promising outcomes and warrants further investigation in a prospective pediatric trial.
Leger: Jazz Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbott Diagnostics: Research Funding. Roth: Merck: Consultancy; Janssen: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal